It is encouraging to know that, according to Cancer Research UK, more people are surviving cancer than ever. Whilst this is good news, it still remains that treatments for cancer often causes a lot of harm and discomfort to the patient, and recovery can be particularly unpleasant. This is due partially to how similar cancer cells are to regular healthy cells and so drug treatments designed to attack these cancer cells will often will also attack the regular cells.
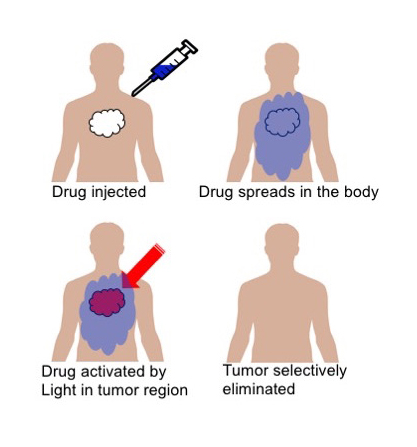
A recent study using the Central Laser Facility's (CLF) Octopus Laser Facility has been aiming to develop better weapons to fight cancer and has revealed some fascinating findings into a possible new cancer treatment that can specifically target tumours, while leaving the rest of the body unaffected. This technique is called Photodynamic Therapy (PDT), and has been in development for a number of years having shown several advantages compared to chemotherapeutic cancer treatment. In particular, is its ability to target cancer at a very specific and localised level via the precise activation of photosensitising drugs illumination directly in the vicinity of malignant cells, while healthy (but similar) cells throughout the patient's body are unaffected by inactive drug molecules.
The research into targeted tumour fighting drugs came from an exciting collaboration involving the CLF, the Scientific Computing Department (SCD) and University of Salford which fashioned a diverse pool of expertise.
Dr. Nicole Holzmann (SCD), a computational scientist involved in this experiment, began her journey into photodynamic therapy via theoretical calculations on how an existing anti-cancer drug could have its properties modified to make it applicable for PDT. These calculations then aided the practical experiment conducted at the CLF, which involved an expert team of microbiologists including Prof. Stan Botchway, Prof. Tony Parker (both CLF) and Prof. Roger Bisby from University of Salford.
Dr. Holzmann said about the experiment, “We were glad that, with our calculations, we were able to show the impact of different modifications to Combretastatin A4. Amongst others, we were able to gain insight into compound properties like the efficiency of the light-induced drug activation and we hope that these findings will be the basis of new experiments at CLF."
In basic terms, the experiment began with a proven to be effective anti-cancer drug, Combretastatin A4 (CA4), and chemically altered to photoreact with a desired wavelength of light that would be most suitable for its purpose. This allowed scientists to alter its conformation, or “switch on", its harmful properties at will, going from a harmless drug to a harmful one. Concluding that, in theory, once the drug is fully administered throughout the body, laser light in the red/near infrared spectrum could be used to “switch on" the drug to its cancer fighting conformation, but only in the area of the body where the cancer is.
“In these novel experiments, we have used two-photon visible or near infra-red excitation (630-900 nm) for the PDT drug activation," Prof. Stan Botchway explained. “This is superior to using one-photon excitation since otherwise one-photon in the UV (320-400 nm) would be needed. The use of NIR (near infrared) light has the advantage that it is less phototoxic and penetrates deeper into tissues. The collaboration with SCD would also allow the development of better multiphoton absorbing combretastatin drugs."
“This initial modification (to NIR) was important," Dr Holzmann added. “Because with different wavelengths you can achieve different degrees of tissue penetration; which you potentially need to do as the tumour might not be located directly under the skin."
With further testing, the team hopes to show that the drug only will attack the laser eradiated area and will leave the rest of the body unharmed due to the way it “latches" onto proteins in the body. Further experiments need to be done on this, but once the molecules in the drug have attached themselves to the proteins, they may not move from there and will eventually be metabolised harmlessly.
Despite being an effective anti-cancer drug, Combretastatin A4 has severe side effects – side effects that could be lessened or eradicated using the PDT approach.
Although more research needs to be done on the use of combretastatins for PDT, the results from this combination of experiment and calculations showed that a positive headway had been made. Prof. Tony Parker and Prof. Stan Botchway, CLF scientist and experts on laser related biological research, formed part of the team that conducted the practical experiment.
Prof. Parker said about the experiment, “Finding new and better ways to fight cancer is a complex task indeed! What Nicole's work has done has shown us all a new way of how to go about things for a long term approach."
Insightful experiments like this are made far more possible by the use of theoretical calculations. Without these, a greater number of practical experiments may need to be conducted through the process of trial and error. Calculations much like Dr. Holzmann's can save time, money and effort by effectively simulating the trial and error process relatively instantly and cheaply through a computer screen.
Dr. Holzmann said, “There are numerous obstacles to overcome and many years until an anti-cancer drug can make it to application. It would be exciting to see if the PDT technique on combretastatin-like drugs enters the clinical study stage in the next years, however, scientifically there are still many steps to take and problems to solve on the way, both experimentally and computationally."
To read more about this research, please go to: https://pubs.rsc.org/en/content/articlehtml/2018/cp/c8cp05375h